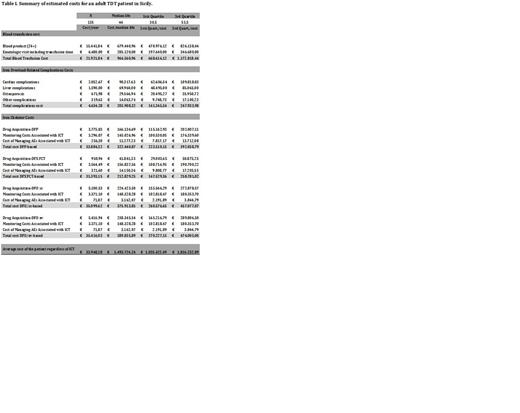
Background: It has been estimated that 5-7% of the world's population carries a mutated gene affecting the production or function of the haemoglobin molecule, and that around 56,000 new individuals affected by Thalassemia Dependent Transfusion (TDT) are born every year worldwide. Innovative treatment (IT), such as gene addition therapy (GT), gene editing (GE) and ineffective erythropoiesis modulating drugs (IEMD), have been developing for the cure of TDT patients, offering chances for obtaining durable transfusion independence (TI). The possibility of bringing IT to the “bed-site” of the patient requires sustainable cost for the worldwide health systems. The accessibility to IT may be different according to the private or public organization of the different Country's health systems, this heterogeneity representing one of the main issues to be solved, even in Countries with high gross domestic product like many of those present in Europe. Therefore, the precise definition of the current costs for TDT lifetime treatment in Countries where thalassemia is common is pivotal for the accessibility of IT to the National Health System. Methods: The main aim of this study was to provide an updated information on the current direct cost of TDT patient management, in a region (Sicily, Italy), where there is a Western Public Funded Healthcare System (WPFHS), high incidence of TDT, with well-organized prevention and treatment program, and accurate control of TDT/birth-rate incidence, implemented from 1983 by the Regional Registry on Thalassemia, for assessing the economic impact of well-organized TDT management in a WPFHS. This monocentric study involved adult TDT patients undergoing regular and intensive treatment, at Campus Hematology Franco and Piera Cutino, AOR Villa Sofia-V. Cervello- Palermo (Italy), from 1980 to today. The estimated costs of management of TDT patients included, besides red blood cell transfusions, staff salary, iron chelation with necessary equipment, side-effects monitoring and complications costs (assumed to be equal in Italy, as estimated by Alta Scuola di Economia e Management dei Sistemi Sanitari, Catholic University of the Sacred Heart, Rome, Italy). Results: The summary of results of calculated direct costs for TDT patients management, estimated on 135 patients, are reported on Table I. The overall cost of the lifetime treatment was calculated for the median life (44 years) of TDT patients in Italy, for the 1 st (30.5 years) and 3 rd quartile (53.5years) of our population, respectively. The blood requirement of 2 blood units, with an interval between transfusions of 15 days, was considered as the standard cost for transfusion treatment. Therefore, the transfusion cost for pediatric patients will be less, while a small cohort of adult patients may have higher cost. Table I shows how the cost for managing complications increases with patient age. The direct cost of oral iron chelators, Deferiprone and Deferasirox, was updated, because chelator patents expired (Tab. I). It is noteworthy to underline how, after oral chelators patents expired, DFO is today the most expensive chelation treatment (Tab. I). The social cost and those related to the loss of working days was not calculated in this study. The three estimation costs may be useful for comparing cost of TDT according to the variation of the median life among different Countries.Conclusion: The cost of TDT patient in WPFHS decreased over the last years, because of the oral iron chelator patents expiring. This cost changes according to age, because of the different incidence of complications, increasing from € 1.035.422,49 (30.5 years) to € 1.816.232,89 (53,5 years)with a median life cost of € 1.493.724,24.These data support the concept that any kind of IT has to be offered at competitive cost in comparison with that of conventional treatment. Finally, considering the permanence of “uncertainty area” for the sustained patient benefit, a reimbursement based on the “pay at result” approach should be considered.
Disclosures
Forni:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Vertex: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Angelucci:Menarini/Stemline: Consultancy; Novartis: Honoraria; bluebird bio, GSK: Membership on an entity's Board of Directors or advisory committees, Other: involvement w/ advisory boards; BMS, Vertex: Other: participation in DMC.